One Dimensional Noble Gas: The actions of atoms has been examined by researchers ever since it was hypothesized that they are the standard systems of the Universe.
The activity of atoms plays an essential duty in essential occurrences like warm, pressure, the motion of fluids, and chemical changes.
Standard spectroscopic methods have the capacity to take a look at the activity of comprehensive clusters of atoms and consequently use averaged details to illuminate occurrences at the atomic level.
However, these approaches don’t show what private atoms are doing at a particular moment.
Physicists encounter difficulties when attempting to image atoms as a result of their tiny size, ranging in between 0.1 and 0.4 nanometers, and there quick movement at speeds of around 400 meters per secondly in the gas stage, which is comparable to tjhe rate of audio.
The problem in directly imaging atoms moving makes it a considerable clinical obstacle to develop continual, real-time visual representations of atoms at work.
“Carbon nanotubes allow us to entrap atoms and precisely position and research them at the single-atom degree in real-time,” stated University of Nottingham’s Professor Andrei Khlobystov.
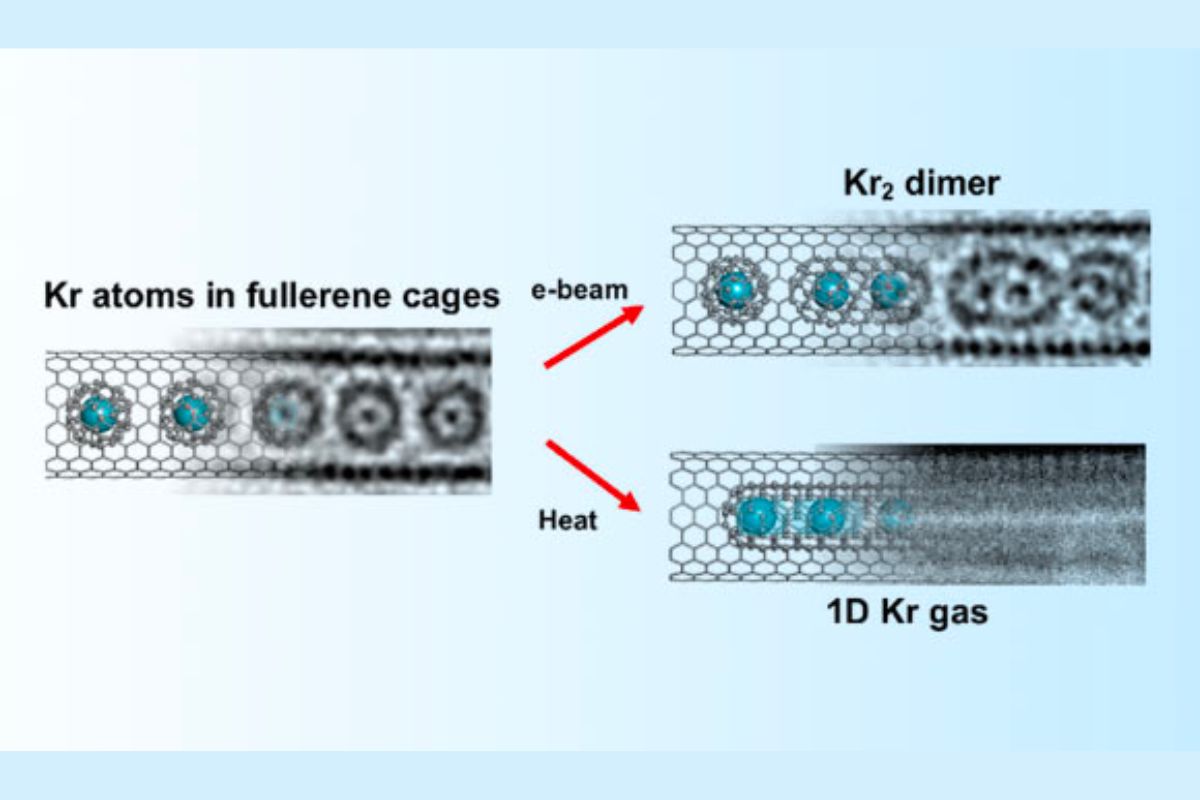
In our study, we had the ability to successfully catch atoms of the honorable gas krypton (Kr).
“Since krypton has a high atomic number, it is less complicated to observe in a TEM than lighter elements. This allowed us to track the positions of krypton atoms as moving dots.”
“We used our state-of-the-art SALVE TEM, which deals with chromatic and spherical aberrations, to observe the procedure of krypton atoms joining together to form krypton sets,” said College of Ulm’s Professor Ute Kaiser.
The eye-catching pressures between particles and atoms in these sets are credited to the van der Waals communication, a sensation that stays enigmatic in the realm of molecular and atomic communications.
“This is an interesting development, as it allows us to see the van der Waals range between 2 atoms in actual room.”
This is a notable development in the realm that can enhance our understanding of the mechanisms of atoms and molecules.
The scientists made use of buckminsterfullerenes, which are molecules formed like footballs and composed of 60 carbon atoms, to carry solitary krypton atoms right into nanotubes for screening purposes.
The combination of buckminsterfullerene molecules to create carbon nanotubes organized in layers played a considerable duty in boosting the precision of the experiments.
According to Ian Cardillo-Zallo, a doctoral candidate at the College of Nottingham, the fullerene tooth cavities can be used to launch krypton atoms by fusing the carbon cages.
“This can be attained by heating at 1,200 degrees Celsius or irradiating with an electron light beam.”
“In a solitary TEM experiment, scientists can examine the communications in between krypton atoms and there one-of-a-kind behavior, which resembles a dynamic gas, permitting a far better understanding of interatomic bonding.”
The scientists had the ability to witness the separation of krypton atoms from fullerene cages, which resulted in the formation of a one-dimensional gas.
After being launched from their carrier particles, krypton atoms are confined to relocating a single instructions within the nanotube channel. This limitation is imposed by the unbelievably limited boundaries of the room.
The atoms in the row of constricted krypton atoms can not pass each other and are forced to reduce, like vehicles in traffic jam.
The scientists recorded the essential phase when separated krypton atoms change to a 1D gas, causing single-atom comparison to vanish in the TEM.
Nevertheless, the activity of atoms withing each nanotube was efficiently tracked utilizing a combination of scanning TEM (STEM) imaging and electron energy loss spectroscopy (EELS), which allowed for the mapping of their chemical trademarks.
“By focusing the electron beam to a diameter much smaller sized than thge atomic size, we are able to check across the examination nanotube and document spectra of individual atoms constrained within, even if these atoms are moving,” claimed Teacher Quentin Ramasse, Supervisor of SuperSTEM, an EPSRC National Research Center.
This offers us with a spooky representation of the gas in one dimension, validating thgat the atoms are dispersed throughout and inhabit any offered location, comparable to how a typical gas acts.
“As for we understand, this is the first time that chains of worthy gas atoms have actually been imaged straight, resulting in the production of a one-dimensional gas in a strong material,” claimed College of Nottingham’s Professor Paul Brown.
“Such strongly correlated atomic systems may show highly unusual warm conductance and diffusion residential properties.”
Transmission electron microscopy has actually contributed in understanding the motion of atoms in real-time and physical space.